Congrès France
Bioproduction
8ème édition
03 & 04 avril 2024
Rassembler et fédérer tous les acteurs au service de la filière

LFB Biomanufacturing
Partenaire Platinium
LFB BIOMANUFACTURING is one of the key entities in the LFB group’s bioproduction activities. Created in France in 1994, LFB group is a leading European company providing plasma-derived medicinal products to healthcare professionals. its mission is to offer patients new treatment options for unmet needs in three major therapeutic areas: immunology, haemostasis and intensive care. LFB’s current market portfolio includes 15 biomedicinal products sold in about 30 countries.
LFB BIOMANUFACTURING harnesses its scientific heritage to help meet patients’ needs globally by providing top decile CDMO services of antibody and therapeutic protein-based therapies. LFB BIOMANUFACTURING operates the Alès (Gard department) industrial site specialising in the manufacture of therapeutic proteins, mainly recombinant proteins and monoclonal antibodies by cell culture. On its FDA-approved industrial site in Alès, LFB BIOMANUFACTURING manufactures a recombinant coagulation factor for the LFB group and acts as a CDMO offering bioproduction services.
LFB BIOMANUFACTURING provides development, manufacturing and analytical services, from pre-clinical to clinical stages. Our development services include cell line development and cell banking, USP and DSP process development and scale-up. Upstream and downstream processes development are executed in state-of-the-art facilities. LFB BIOMANUFACTURING’s USP technological options include the ambr®15 and ambr®250 advanced microscale bioreactor system for media/feed optimization and single use bioreactors such as wave or disposable stirred tank systems for seed train / scale-up definition. Our scientists and experts will facilitate the development of optimum cell culture conditions for each cell line. Our engineering staff possess strong experience and technical knowledge for the selection of optimal chromatography and filtration/purification processes and viral clearance methods. LFB BIOMANUFACTURING can also rely on intra LFB group extensive on pharmaceutical development activities such as formulation development.
LFB BIOMANUFACTURING has a proven track record in the manufacturing antibodies and recombinant proteins including non-GMP batches up to 50L in pilot area and GMP Batches up to 1000L in one of the 3 multi-purpose workshops – additional capacities up to 6x 2000L are being installed.
Our experienced analytical team applies a broad range of methods to support process development and product release. Our laboratory is fitted with state-of-the-art equipment to enable us to provide advanced characterization of your product.
For DP manufacturing, LFB BIOMANUFACTURING has chosen to partner with well-known pharmaceutical companies with long expertise in fill and finish activities, while LFB remains on the top of your project by keeping its E2E view from DNA to patient.
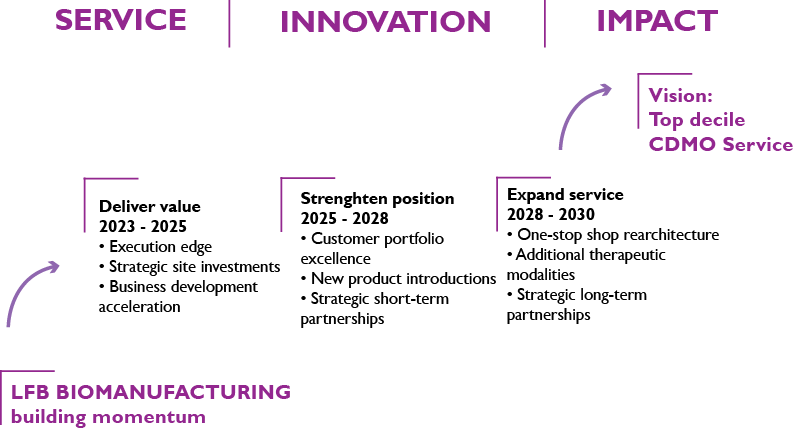
ESCAPE30 OUR NEW DEVELOPMENT STRATEGY TOWARDS BEING A TOP DECILE CDMO FOR BIOLOGICS
In November 2022 the management team of LFB BIOMANUFACTURING met to define the new strategic path of the company, referred to as ESCAPE30, and its associated long-term priorities.
LFB BIOMANUFACTURING targets Excellence in Services as CDMO for monoclonal Antibodies and therapeutic Proteins in Europe by 2030 – this will contribute to the national sovereignty in the field.
ESCAPE30 is a “France 2030” winner. Indeed, in response to the “2030 Industrialisation and Health Capacity” call for projects, the French Prime Minister decided to fund ESCAPE30 for up to €6.7 million which is 30% of the total project cost.
ESCAPE30 strategic investments include (and not limited to) a USP capacity increase with acquisition of 2x2000L disposable bioreactors, new process development and analytical services labs with state-of-the art equipment as well as several new facilities (warehouse, media & buffer area, additional DSP suite). ESCAPE30 will significantly increase the capability of LFB BIOMANUFACTURING resulting in the ability to handle 4 additional biotech, start up and mid-size pharma projects per year.
ANALYTICAL METHOD DEVELOPMENT: A UNIQUE IN-HOUSE EXPERTISE FOR BIOLOGICS
Our local analytical team has the expertise to handle method transfer, method development and validation to have the most robust process to ensure the success of the cGMP manufacturing of your drug substance and drug product.
The routine activities include but not limited to:
– Raw material testing and EM,
– Method development and validation,
– Analytical support for USP and DSP in-process controls,
– DS testing and release for all clinical phases and up to commercialization,
– DS and DP Stability studies.
– Our specific analytical package for MAbs/proteins includes a range of methods among which:
– MAB and total protein quantification (affinity chromatography and OD280),
– Purity by capillary electrophoresis (cGE) and size exclusion chromatography,
– Charge variants by cIEF and CEX-HPLC,
– Process related impurities: Protein A, HCP and DNA,
– Custom biological activity by ligand binding assay (ELISA),
– Endotoxins and bioburden assessment,
– Excipient quantification (i.e. polysorbates by HPLC-ELSD),
– Mass spectrometry and peptide mapping for structural characterization and investigational purposes,
Glycan analysis by HPLC-HILIC-FLD.

La France s’est fixé pour priorité d’assurer sa souveraineté sanitaire. Pour y arriver, elle doit regagner son leadership européen et accélérer la transition de son industrie (bio)pharmaceutique. Seule une action collective, permettra de relever ces défis.
Polepharma structure la filière industrielle (bio)pharmaceutique française et accompagne sa transformation depuis 20 ans.
POLEPHARMA
21 rue de Loigny la Bataille
28000 Chartres
Tél. : +33 (0)2 37 20 99 90
Mail : contact@polepharma.com

Le pôle Medicen fédère un réseau d’excellence en innovation sur le territoire francilien et au niveau national. En rassemblant acteurs privés et publics autour des enjeux d’innovation pour développer les solutions thérapeutiques de demain, Medicen est le tiers de confiance de la filière, au service du développement et de l’aboutissement des projets.
MEDICEN
130 Rue de Lourmel
75015 Paris
Tél. : +33 (0)1 79 68 10 87
Mail : medicenparisregion@medicen.org